
sales@labctl.in
+91-9999101371, 011-42154411,
011-45686600, 011-28114400/11/22
WH-52 & A-33, Mayapuri Industrial Area, Ph-1, Delhi-64
Send us your requirements
Inquiry Form

Please fill below inquiry form to help us understand your requirements in details.
Product Categories
Central Drugs Standard Control Organisation (CDSCO)
Central Drugs Standard Control Organisation (CDSCO)
The Central Drugs Standard Control Organisation (CDSCO) under Directorate General of Health Services, Ministry of Health & Family Welfare, Government of India is the National Regulatory Authority (NRA) of India. It’ s vision is “To Protect and Promote public health in India”. Mission is “To safeguard and enhance the public health by assuring the safety, efficacy and quality of drugs, cosmetics and medical devices”.
CDSCO has initiated a program for regulating quality of Medical Devices in a phased manner.
Besides test report from registered / recognised labs, Manufactures are also required to have quality system certified as per IS 13485
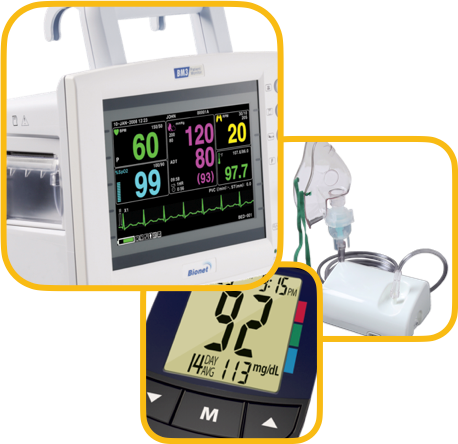
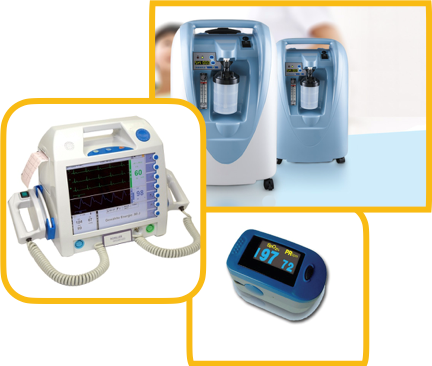
Conformity Testing Labs is the first Lab in India registered with CDSCO for testing the electro medical devices to notified standards. Whereas we can test a wider range of Medical Devices, current Scope of registration under CDSCO include….
Testing Facilities with us Include
| S. No | Products | IS/IEC Standards |
|---|---|---|
| 1. | Nebulizer | Class C |
| 2. | NIBP | Class B |
| 3. | IBP | Class C |
| 4. | Glucometer | Class B |
| 5. | Thermometer | Class B |
| 6. | CT Scan Equipment | Class C |
| 7. | MRI Equipment | Class C |
| 8. | Defibrillators | Class B |
| 9. | PET Equipment | Class C |
| 10. | X-Ray Machine | Class C |
| 11. | Dialysis Machine | Class C |
| 12. | Bone marrow cell separator | Class C |
Contact
WH-52, Mayapuri Industrial Area Phase-I, New Delhi-110064
011-28114400/ 28114411/ 28114422
A-33, Mayapuri Industrial Area Phase-I, New Delhi-110064
011-45686600